近日,Journal of Molecular and Cellular Cardiology(分子与细胞心脏学期刊)在线发表了来自复旦大学中山医院的研究人员在树突状细胞外泌体激活T细胞改善心梗后心功能的最新发现。
CD4+ T细胞的活化在促进心肌梗死(MI)后伤口愈合的过程中起关键作用。树突细胞(dendritic cells,DCs)分泌的外泌体(Exosomes,EXs)在肿瘤模型中激活T细胞;然而,树突细胞来源的外泌体(dendritic cells derived exosomes,DEXs)是否可通过CD4+ T细胞的活化促进心肌梗死(MI)后伤口愈合仍然不清楚。这项研究旨在确定DEXs是否介导的CD4+ T细胞活化,改善小鼠心梗后的心功能。来自复旦大学中山医院的研究人员使用缺氧的原代或坏死的HL-1心肌细胞的上清液模拟体外梗死后心肌细胞的微环境。用正常(对照组)、缺氧或坏死的HL-1心肌细胞(MI组)的上清液处理小鼠骨髓来源的DCs,以及未刺激(阴性组)。然后从BMDC上清中分离DEXs,孵育CD4+ T细胞或用于小鼠尾静脉注射。在这项研究中,研究者发现,无论是缺氧还是坏死的HL-1心肌细胞的上清均可上调DC成熟的相关基因。注射DEXs后,大量的MI-DEXs被代谢到脾脏,且比对照组和阴性组更快速。共聚焦成像和流式细胞术显示,相比对照组和阴性组-DEXs,MI-DEXs会更容易被脾CD4+ T细胞获取;并且体外与体内结果都显示脾CD4+ T细胞摄取MI-DEXs会表达更多的趋化因子和炎症因子IFN-γ和TNF。此外,注射MI-DEXs改善小鼠MI后的心功能。这些结果表明,DEXs可通过一种内分泌机制介导的CD4+ T细胞的活化,改善MI后心功能。该发现为通过全身递送DEXs治疗MI的新策略提供了基础。
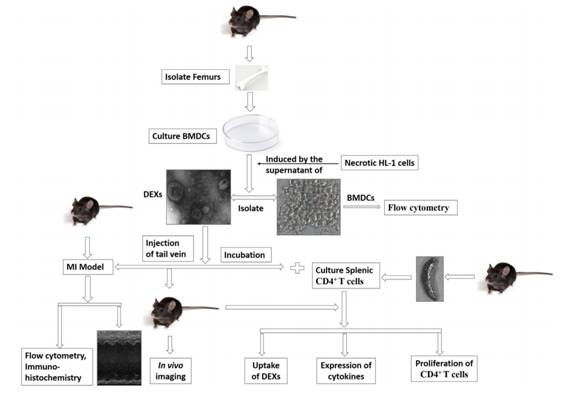
该研究的设计流程如上图。首先分离培养小鼠骨髓来源的DCs,然后用低氧处理和坏死的HL-1细胞的细胞上清处理DCs,然后分离该DCs培养上清中的DEXs,然后用DEXs分别于体外、体内处理心梗后的小鼠和培养的脾CD4+ T细胞,检测小鼠心功能改善情况,和T细胞对DEXs的摄取情况和基因表达的改变。
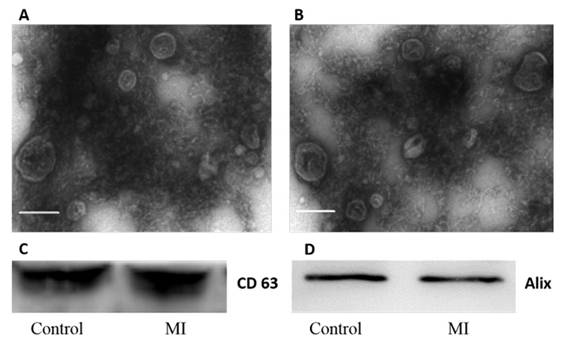
首先对分离得到的外泌体进行了鉴定,分别做了电镜检测和western检测。
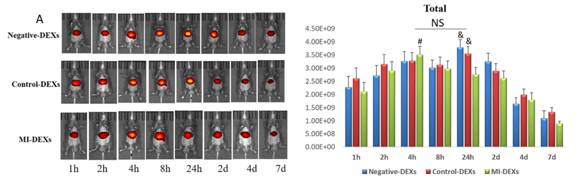
尾静脉注射外泌体后进行活体成像,结果显示三组并无差异。
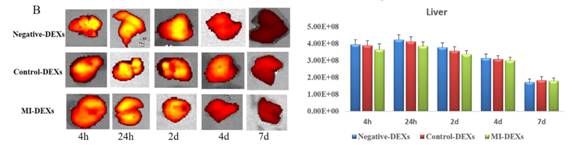
接着检测肝脏的外泌体摄取情况,也并无差异。
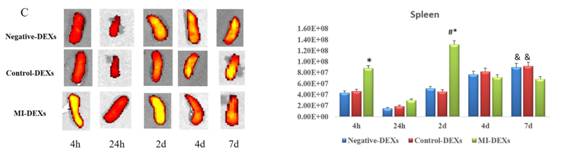
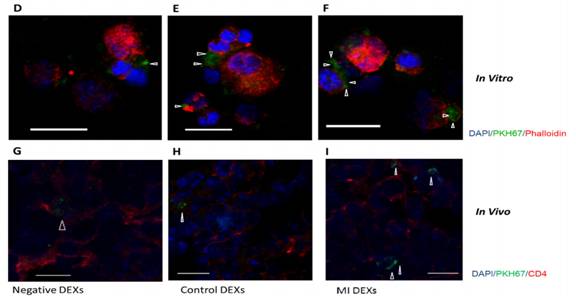
再检测脾脏的外泌体摄取情况,发现脾脏对MI-DEXs的摄取较对照组和阴性组快且多。
通过PKH67标记外泌体检测处理CD4+ T细胞和尾静脉注射24h后的摄取情况。
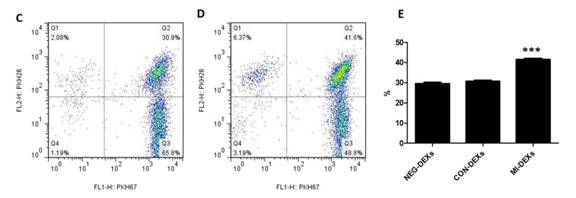
接着,通过流式定量检测CD4+ T细胞对三组DEXs摄取的情况,结果显示MI-DEXs被摄取得最多。
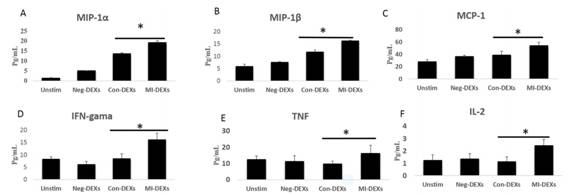
Con-DEXs和MI-DEXs处理CD4+ T细胞后均可促进MIP-1α、MIP-1β、MIP-1、IL-2的分泌,而仅MI-DEXs处理CD4+ T细胞后促进趋化因子和炎症因子IFN-γ和TNF的分泌。
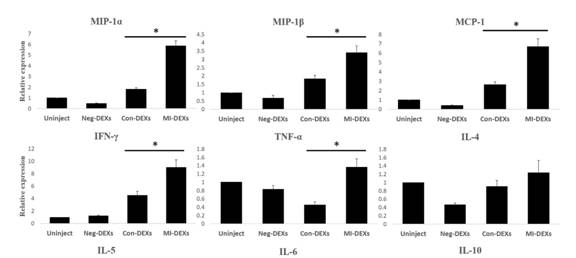
尾静脉注射外泌体后检测脾CD4+ T细胞的趋化因子和炎症因子表达情况。结果显示MI-DEXs处理促进MIP-1α、MIP-1β、MIP-1、IFN-γ和TNF的表达。
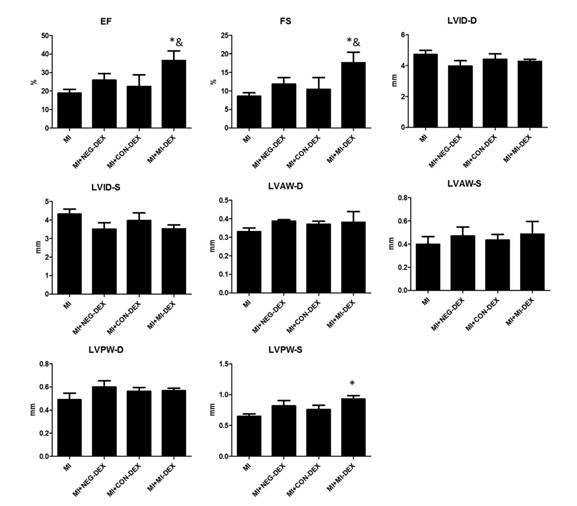
最后分析,尾静脉注射外泌体后对小鼠心梗后心功能的改善情况。结果显示,注射MI-DEXs组在射血分数、左室短轴缩短率和左室后壁厚度3项心功能指标又明显改善作用。
总结,除了已经报道的胚胎干细胞、血浆、间充质干细胞、心血管前体细胞和成纤维细胞的外泌体对心梗具有保护作用,该研究发现树突状细胞(缺氧或坏死的HL-1心肌细胞培养上清预处理)的外泌体也具有心梗保护作用,补充了外泌体治疗心梗的细胞来源,为全身递送外泌体治疗心梗的新策略提供了新的实验基础。
参考文献:
Haibo, L., et al. (2015). "Exosomes derived from dendritic cells improve cardiac function via activation of CD4 T lymphocytes after myocardial infarction." J Mol Cell Cardiol.
CD4+ T cell activation plays a key role in facilitating wound healing after myocardial infarction (MI). Exosomes (EXs) secreted from dendritic cells (DCs) can activate T cells in tumor models; however, whether DEXs (DC-EXs) can mediate CD4+ T cell activation and improve wound healing post-MI remains unknown. This study sought to determine whether DEXs mediate CD4+ T cell activation and improve cardiac function post-MI in mice. We used supernatants of hypoxic primary or necrotic HL-1 cardiomyocytes to simulate the post-MI cardiomyocyte microenvironment in vitro. Cultured bone marrow-derived DCs (BMDCs) from mice were stimulated with the supernatants of normal (Control group), hypoxic primary or necrotic HL-1 cardiomyocytes (MI group); a subset of BMDCs remained unstimulated (Negative group). DEXs were then isolated from the BMDC supernatants and either incubated with CD4+ T cells or injected into mice via the tail vein. In this study, we found that the supernatants of both hypoxic primary and necrotic HL-1 cardiomyocytes upregulate DC maturation markers. After the injection of DEXs, a greater number of MI-DEXs are recruited by the mouse spleen and with greater rapidity than control- or negative-DEXs. Confocal imaging and flow cytometry revealed that MI-DEXs exhibited higher uptake by splenic CD4+ T cells than the control- and negative-DEXs, and this increase was correlated with significantly greater increases in the expression of chemokines and the inflammatory cytokines IFN-gamma and TNF by the CD4+ T cells in vitro and in vivo. In addition, the injection of MI-DEXs improved cardiac function in mice post-MI. These results suggest that DEXs could mediate the activation of CD4+ T cells through an endocrine mechanism and improve cardiac function post-MI. Our findings provide the basis for a novel strategy for the treatment of MI through the systemic delivery of DEXs.
版权归外泌体资讯网所有,欢迎转载,但请注明出处和原文链接!
外泌体资讯网 JMCC:树突状细胞外泌体激活T细胞改善心梗后心功能