随着靶向给药领域的扩大,纳米技术在近几十年来为智能载体的发展做出了重大贡献。特别是,基于脂质的纳米载体为药物封装提供了一个通用平台,这导致了几种制剂的临床转化。除了合成纳米载体外,细胞衍生的基于细胞外囊泡(EVs)的载体系统也引起了相当大的兴趣。
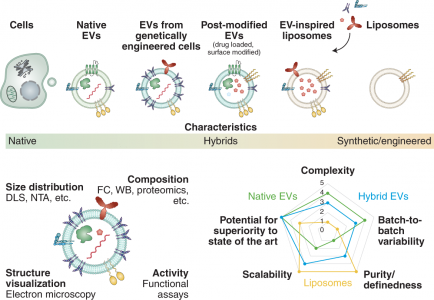
EVs是一组异质的脂质结合纳米颗粒,充当许多(病理)生理过程的关键介质。人们还正在探索利用其固有的组织归巢能力将治疗有效载荷输送到特定细胞或组织。从药物递送的角度来看,EVs与脂质体相当,因为两者都是基于磷脂的。然而,EVs是由各种脂质、表面和膜蛋白的复杂混合物组装而成的。其中一些组件有助于组织靶向,而其他组件则确保将非特异性相互作用降至最低。这些独特的蛋白质修饰的磷脂囊泡已被假定包含特定“条形码”在局部和远距离寻找所需的靶标。尽管进行了广泛的研究,但基于EVs的药物递送优于通过工程纳米载体(如脂质体)递送的优势以及相关的风险收益比仍然存在争议。
该综述批判性地讨论了EVs作为药物输送载体和下一代疗法的前景。概述了EVs相对于标准递送方法的优势,讨论了与其临床和工业转化相关的当前障碍,并强调了与其他新兴领域的协同作用,例如细胞疗法(EV有时被认为是“无细胞的细胞疗法”)。还提出了关于实验要求和科学需求的指南,以促进EVs作为药物载体的发展,以评估其递送功效并允许对替代品进行基准测试。
该综述大体包含如下内容:
- EV生物学和功能的独特性
- EVs的组成成分
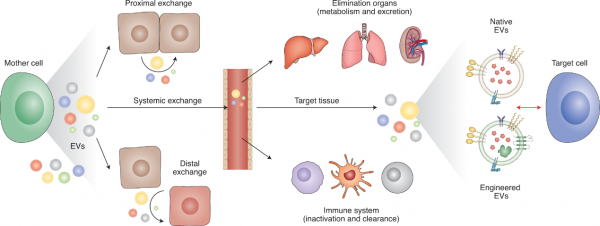
- EVs的吸收和生物学作用
- 基于EVs的药物载体
- 相关开发
表1 . 正在进行的EVs临床试验
| No. | 试验名称 | 状态 | 适应症 | EV的类型 | 坐标 | NCT号 |
| Stem-cell-derived EVs | ||||||
| 1 | A Clinical Study of Mesenchymal Progenitor Cell Exosomes Nebulizer for the Treatment of Pulmonary Infections | Recruiting; phase 1/2 | Drug-resistant infections | MSC-/progenitor-cell-derived exosomes | Shanghai, China | NCT04544215 |
| 2 | Effect of Microvesicles and Exosomes Therapy on β-Cell Mass in Type I Diabetes Mellitus (T1DM) | Unknown status | Diabetes mellitus type 1 | MSC-derived exosomes | Sahel, Egypt | NCT02138331 |
| 3 | Evaluation of Safety and Efficiency of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia | Enrolling by invitation; phase 1/2 | SARS-CoV-2 pneumonia | MSC-derived exosomes | Samara, Russia | NCT04491240 |
| 4 | A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes for the Treatment of Severe Novel Coronavirus Pneumonia | Completed; phase 1 | SARS-CoV-2 pneumonia | MSC-derived exosomes | Shanghai, China | NCT04276987 |
| 5 | Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia | Enrolling by invitation; phase 2 | SARS-CoV-2 pneumonia | MSC-derived exosomes | Samara, Russia | NCT04602442 |
| 6 | Effect of UMSCs Derived Exosomes on Dry Eye in Patients With cGVHD | Recruiting; phase 1/2 | Dry eye | Umbilical MSC-derived exosomes | Guangzhou, China | NCT04213248 |
| 7 | MSC-Exos Promote Healing of MHs | Recruiting; early phase 1 | Macular holes | MSC-derived exosomes | Tianjin, China | NCT03437759 |
| 8 | A Tolerance Clinical Study on Aerosol Inhalation of Mesenchymal Stem Cells Exosomes in Healthy Volunteers | Recruiting; phase 1 | Safety and tolerance studies | MSC-derived exosomes | Shanghai, China | NCT04313647 |
| 9 | Allogenic Mesenchymal Stem Cell Derived Exosome in Patients with Acute Ischemic Stroke | Completed; phase 1/2 | Cerebrovascular disorders | Mesenchymal-stromal-cell-derived exosomes | Tehran, Iran | NCT03384433 |
| 10 | Evaluation of Adipose Derived Stem Cells Exosomes in Treatment of Periodontitis | Recruiting; early phase 1 | Periodontitis | Adipose-derived stem-cell-derived exosomes | Cairo, Egypt | NCT04270006 |
| Allogenic and autologous EVs | ||||||
| 11 | Safety and Efficacy Evaluation of Allogenic Adipose MSC-Exosomes in Patients with Alzheimer’s Disease | Recruiting; phase 1/2 | Alzheimer’s disease | Allogenic adipose MSC-derived exosomes | Shanghai, China | NCT04388982 |
| 12 | A Clinical Study of Mesenchymal Stem Cell Exosomes Nebulizer for the Treatment of ARDS | Not yet recruiting; phase 1/2 | Acute respiratory distress syndrome | Allogeneic human MSC-derived exosomes | Ruijin, China | NCT04602104 |
| 13 | MSC Extracellular Vesicles in Dystrophic Epidermolysis Bullosa | Not yet recruiting; phase 1 | Dystrophic epidermolysis bullosa | Allogeneic MSC-derived EVs | Aegle Therapeutics | NCT04173650 |
| 14 | Effect of Plasma Derived Exosomes on Cutaneous Wound Healing | Enrolling by invitation; early phase 1 | Ulcer | Autologous exosome-rich plasma | Kumamoto, Japan | NCT02565264 |
| Other cells or EV sources | ||||||
| 15 | COVID-19 Specific T Cell Derived Exosomes | Active; phase 1 | SARS-CoV-2 pneumonia | T-cell-derived exosomes | Kayseri, Turkey | NCT04389385 |
| 16 | Extracellular Vesicle Infusion Therapy for Severe COVID-19 | Not yet recruiting; phase 2 | SARS-CoV-2 pneumonia, acute respiratory distress syndrome | Bone-marrow-derived EVs | Direct Biologics | NCT04493242 |
| 17 | Edible Plant Exosome Ability to Prevent Oral Mucositis Associated with Chemoradiation Treatment of Head and Neck Cancer | Active; phase 1 | Head and neck cancer, oral mucositis | Grape exosomes and fentanyl patch | Louisville, USA | NCT01668849 |
| Drug-loaded EVs | ||||||
| 18 | iExosomes in Treating Participants with Metastatic Pancreas Cancer with KrasG12D Mutation | Recruiting; phase 1 | Metastatic pancreatic adenocarcinoma, pancreatic ductal adenocarcinoma | Mesenchymal-stromal-cell-derived exosomes loaded with siRNA against KrasG12D | Houston, USA | NCT03608631 |
| 19 | Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue | Active; phase 1 | Colon cancer | Plant exosomes loaded with curcumin | Louisville, USA | NCT01294072 |
| 20 | Trial of a Vaccination with Tumor Antigen-loaded Dendritic Cell-derived Exosomes | Completed; phase 2 | Non-small-cell lung cancer | Dendritic-cell-derived exosomes loaded with antigen | Villejuif, France | NCT01159288 |
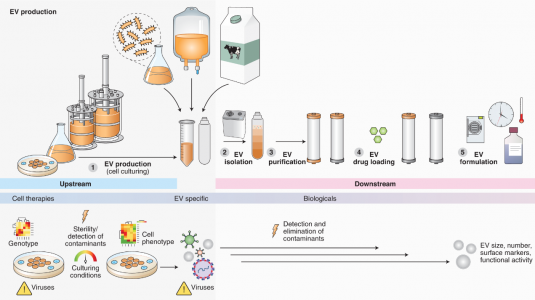
- 关键单元操作:上游
- EVs来源的细胞培养和表征
- 可选的内源性载药
- EVs收集和工程化
- EVs的分离和表征
- 外源载药
- 下游
- 纯化
- 质量控制——工程EVs的(最小)表征
- 制剂和保质期
- 安全源于设计和流程去风险化
最后观点
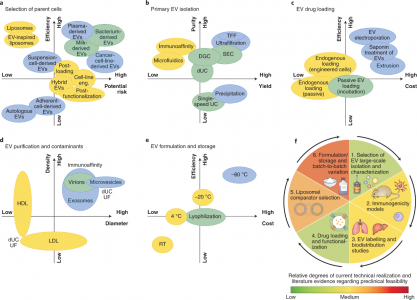
EVs可用作各种药物递送应用的载体系统。与标准递送方法相比,EVs已被证明在系统性给药于啮齿动物时,递送的功能性货物免疫清除率低。然而,需要在临床相关系统中进行更多评估,该文与基于脂质体的替代品进行直接、定量的比较,以全面评估风险收益比。EVs的成功转化取决于具有成本效益的大规模生产、分离和表征方法的可用性,该方法具有高灵敏度以评估批次间的差异(及其生物学后果),以及广泛适用的装载药物方法的可用性。新分析技术的日益普及有望为EVs的独特性提供新的见解,并可能激发下一代合成系统的工程设计。人造EVs或EVs模拟物的生产可以克服与无菌、大规模生产和监管相关的挑战。人们已经在探索令人兴奋的新途径,包括将载药脂质体与EVs融合以提高载药能力。值得注意的是,最近报道了通过植入细胞生产设计的EVs。该技术为工程外泌体的体内生产提供了一条新途径。尽管取得了这些有希望的结果,但仍需要更多地了解使EVs如此有效地渗透细胞和逃避免疫检测的机制,以充分发挥其潜力。